DESKTOP DIGITAL SPIROMETER
DESKTOP DIGITAL SPIROMETER Specification
- Noise Level
- 0 db
- Color Code
- BLACK
- Material
- PVC
- Application
- SPIROMETORY TEST
- Product Type
- PORTABLE
- Weight
- 75 GSM (gm/2)
- Real-Time Operation
- REAL TIME Seconds
DESKTOP DIGITAL SPIROMETER Trade Information
- Minimum Order Quantity
- 1 Piece
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 1000 Pieces Per Week
- Delivery Time
- 4 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Africa, Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East
About DESKTOP DIGITAL SPIROMETER
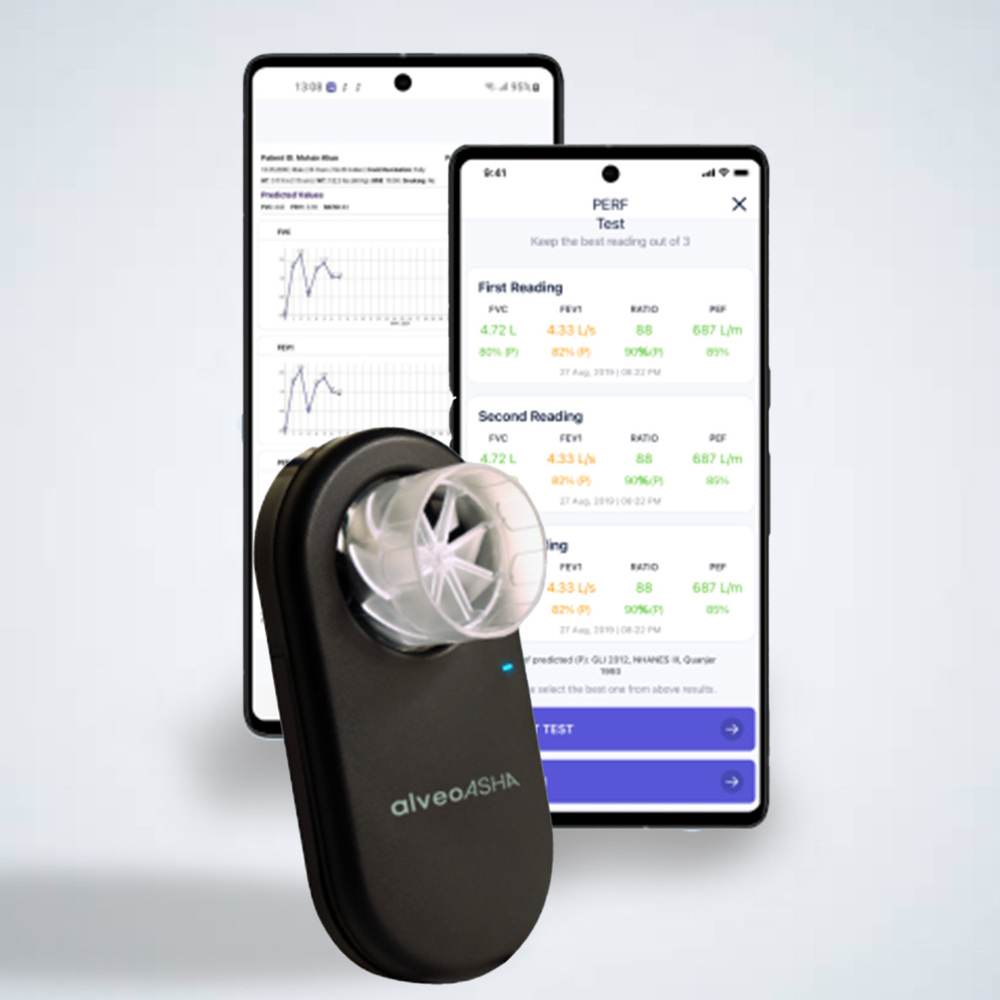
Device Name
alveoair
Medical Device Class
Class B
Data Storage
Cloud Storage
Sensor Technology
Accu-sense proprietary Optical interference
Flow Range
0-14 L
Test Modes
Standard, Full Loop, and Pre-Post bronchodilator
Volume Accuracy
2.5% or 75 ml whichever is greater
Dimensions (H-L-W)
H-110 cm. L-56| cm, W-18 mm
Power Supply
AAA Batteries
Wireless Connection
BLE 5.4
Use
Offices, Clinics, Occupational
Environment
Health centres, Ambulances, Primary Care centres, Hospitals
Weight (With batteries)
76 gm
Mouthpiece
Standard Turbine 28 mm
Predicted Values
Quanjer 2012 (GU), NHANES IIII 1999 (Hankinson), ERS 1993
Interface Device
Android Mobile phone above Android OS 8
Calibration
No Calibration is required. (Precalibrated)
Reverse Flow Detection
Yes
ATS Error Codes
Lower Limits of Normal (LLN & Zscore Implementation
Quality / Medical Devices / Electrical Safety Standard
ISO-26782:2009, ISO-23747:2015, IEC 60601-1, IEC 60601-1-11 IEC 60601-1-2, ISO-10993-1, ISO-10993-5, ISO-10993-10, ISO-10993-23, Roundw-Pune-MH/M/ MD/001068, FCC ID 2AV9TD8-52832-01
Parameters
FVC, FEVL FEV1/FVC, PEF, MEF25 (FEF75), MEF25 (FEF75), MEFSO (FEF50), MEF75 (FEF25), FEF25-75, Duration Exhale, Lung age, FIVC, PIF, MIF75, MIF50, MIF25, MIF50/MEF50, Duration Inhale, BEV, FEFIO, FEF25-75/FVC, FEF40, FEF50/FVC, FEF60, FEF80, FEV 25, FEV.50, FEV.75, FEV.50/FVC, FEV.75/FVC, FEV3 FEV3/FVC, MEF95



Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Respiratory Instrument Category
Laryngoscope Set Neonate
Price 1950 INR / Piece
Minimum Order Quantity : 10 Pieces
Material : Mild steel
Portable : Yes
Use : Hospital
Condition : New
Peak Flow Meter And Mouthpieces
Price 400 INR / Piece
Minimum Order Quantity : 10 Pieces
Material : Plastic
Portable : Yes
Use : Hospital
Condition : New

 Send Inquiry
Send Inquiry


